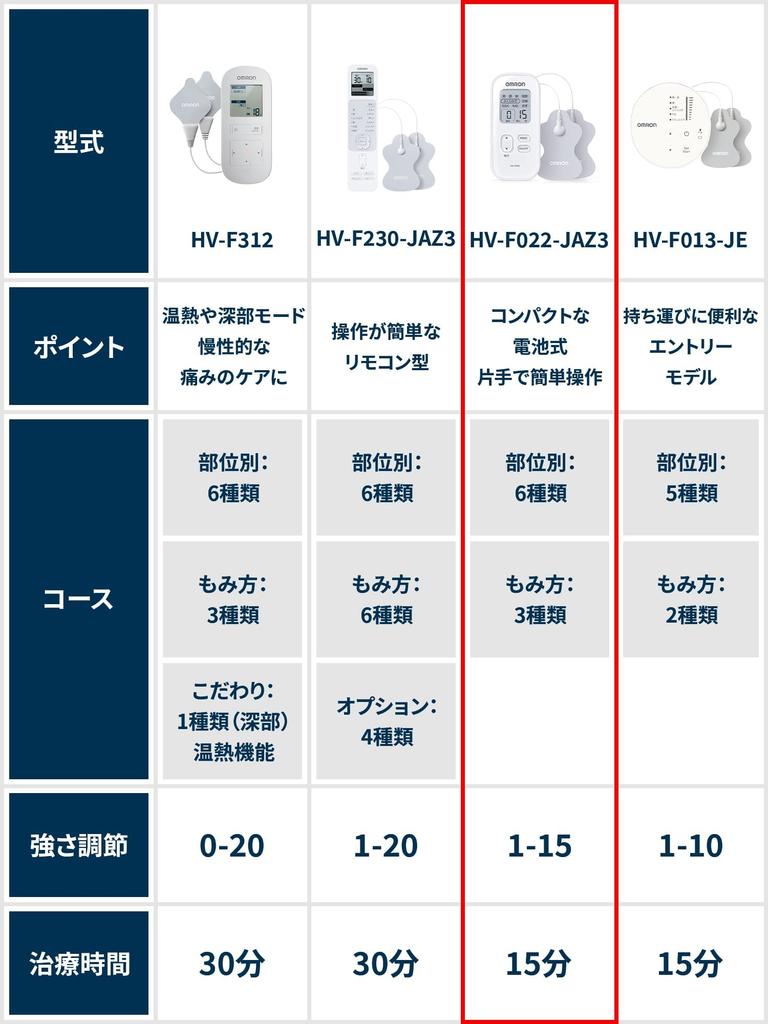
Omron Low Frequency Therapy Device HV-F022-JAZ3 White










Beskrivelse
[Please be sure to read the instruction manual]
[Generic Name] Home Low-Frequency Therapy Device
[Product Name] Omron Low-Frequency Therapy Device HV-F022-JAZ3
[Warning] The following individuals should consult a doctor before use: (1) Those currently receiving medical treatment or those experiencing any physical abnormalities (2) Those with malignant tumors (3) Those with cardiac or neurological abnormalities (4) Pregnant women or those who have just given birth (5) Those with a body temperature of 38°C or higher (fever stage) (Example 1: Those experiencing severe acute symptoms (fatigue, chills, blood pressure fluctuations, etc.) Example 2: Weakness (6) People with infectious diseases (7) People with skin sensory impairments or other skin abnormalities (8) People with acute (painful) illnesses, such as spinal fractures, sprains, or pulled muscles (9) People requiring rest (10) People taking medication (11) People with abnormal blood pressure (12) People with limb disabilities (13) People unable to express themselves (14) People with sensory impairments due to severe peripheral circulatory disorders such as diabetes (15) People with low temperatures (16) People with poor circulation (17) People who do not see results even after using the device for a while (18) People with skin discomfort caused by pads (19) People with metal implants • Continued use may result in an accident or poor health.
Do not allow people unable to express themselves or who require assistance to use the device alone.
• It may result in an accident, injury, or poor health. Do not use near the heart, chest, above the neck (including the head and mouth), genitals, or areas with skin diseases. Also, do not use in a way that pinches internal organs. • Doing so may result in an accident or ill health. Do not use in conjunction with other treatment devices, two or more of these products at the same time, or in conjunction with medications such as compresses, liniments (including sprays), or ointments. • Doing so may result in ill health or ill health. Do not use for any purpose other than treatment. Do not use if the surface of the pad is chipped or broken. Do not use if the pad has peeled off. • Doing so may result in an accident, malfunction, or breakdown. Do not insert the lead cord insertion plug into any location other than the lead cord insertion port on the main unit. • Doing so may result in electric shock or an accident. Do not bend or fold the pads forcefully. • Doing so may result in electric shock or an accident. Do not use if the lead cord is damaged, such as a broken cord or a damaged plug. Purchase this item separately. • May cause electric shock, short circuit, or fire.
Do not allow children to use the device or play with the device or pad. Also, do not allow children to climb on it.
• May cause accidents, injuries, ill health, or malfunctions.
Do not use in humid areas or while bathing.
• May cause electric shock.
Do not use while sleeping.
• May cause malfunction. The pad may also stick to unexpected places, causing ill health.
Do not use while driving a car or operating dangerous machinery.
• Strong stimulation may cause an accident or malfunction.
Do not disassemble, repair, or modify the device.
• May cause fire, malfunction, or accidents.
[Contraindications/Prohibitions] Never use this device in conjunction with the following medical electrical equipment. (1) Implantable medical electrical devices such as pacemakers (2) Life-sustaining medical electrical devices such as heart-lung machines (3) Wearable medical electrical devices such as electrocardiographs • This may cause these medical electrical devices to malfunction, resulting in serious harm to life.
[Product Specifications] Power Supply: DC 3V (Uses two AAA alkaline batteries) Battery Life: Approximately 4 months (based on 15 minutes of continuous use once per day) Service Life*: Approximately 5 years (based on self-certification (company data)) *Based on standard usage (15 minutes per day) Maximum Output Current: 10mA or less Fundamental Frequency: Approximately 1-238Hz Rated Output Voltage: Maximum 80V Maximum Pulse Width: 150μsec Power Consumption: Approximately 0.1W Rated Time: 15 minutes Operating Environment: +10-+40°C, 30-85% RH (non-condensing), 700-1060hPa Storage Environment (after opening): 0-+40°C 30-85% RH (no condensation) Weight: Approx. 100g (including batteries) Dimensions: 112mm x 52mm x 25mm Operating Principle: Utilizing the nerve and muscle response to low-frequency electricity, a weak pulse current is passed through the skin surface, resulting in physiological effects that treat the affected area. Accessories: Electrode Cord Type B (White) (1 piece), Long Life Pad HV-LLPAD-GY (Gray) (1 set of 2 pads) Product Name: Long Life Pad HV-LLPAD Manufacturer/Distributor: Sekisui Chemical Co., Ltd. Manufacturer/Distributor Notification Number: 08B2X10006000007 Two AAA alkaline batteries (trial size), Electrode Storage Organizer (Gray) (1 piece), Instruction Manual (Quality Guarantee Certificate included) Composition of parts that come into direct contact with the body: Acrylic resin, glycerin, water Sold Separately: Long Life Pad (Gray), Electrode Cord Type B (White)
[Usage Precautions] Apply the pads correctly. • Failure to do so may result in an accident or poor physical condition. Make sure the electrode cord, device, and pad are properly and securely connected before use. • Improper connection may result in electric shock, accident, or malfunction. If you experience any physical abnormalities or skin irritations such as eczema, redness, or itching, discontinue use immediately. • Consult a doctor and follow their instructions. If you need to change pads to a different part of the body during treatment, be sure to turn off the power before doing so. • You may be subjected to a strong shock. If the device stops working or you notice any abnormalities, turn off the power immediately and contact the Omron Customer Service Center. • Doing so may lead to accidents, problems, or malfunctions due to overheating or a short circuit. When removing the lead cord from the main unit, always hold the lead cord plug and unplug it, not the lead cord itself. • Doing so may result in electric shock, accidents, or malfunctions. When applying the pads, be careful not to get the lead cord entangled around your neck. • Doing so may result in accidents or injury. If the adhesive strength of the pads decreases, clean them. If the adhesive strength of the pads persists, purchase new pads separately. • Continued use may result in accidents or poor health. After washing the pads with water, dry them thoroughly before using them again. • Doing so may result in electric shock or an accident. Store the pads attached to the electrode storage device. Always keep the pads clean when storing them. • Improper storage may cause damage to the pads. Do not leave the pads attached to the affected area. • Doing so may cause skin inflammation. Do not use the pads while they are in contact with metal objects such as belts or necklaces. • You may receive a strong shock. Do not use a mobile phone near the low-frequency therapy device. • Doing so may cause malfunctions. Do not use for long periods of time (more than 30 minutes). • Doing so may cause muscle fatigue, ill health, or severe pain. Do not share pads with others. • Doing so may result in infection or skin inflammation. Do not plug in/unplug the cord or attach the pads with wet hands. Do not attach pads to wet areas. • Doing so may result in electric shock or an accident. When using this product, only use products designed for it. • Doing so may result in an accident or malfunction.
[Name of Manufacturer/Distributor] Manufacturer: Omron Healthcare Co., Ltd. 53 Kunotsubo, Terato-cho, Hyuga-shi, Kyoto 617-0002 Tel: 0120-30-6606 (Omron Customer Service Center)
[Purpose of Use or Effects/Efficacy] Relieves stiff shoulders, prevents atrophy of paralyzed muscles, and provides a massage effect. For general household use. Medical Device Classification: Specially Controlled Medical Device Medical Device Certification (Approval) Number: 306AHBZX00018000 Dimensions: 112mm x 52mm x 25mm Power Supply: 3V DC (Uses two AAA alkaline batteries) Weight: Approximately 100g (including batteries)